Hence, the aim of treatment for MM is to induce and maintain remission for as long as possible, thereby increasing the length of survival. Care of patients with MM is complex and focuses on treating the disease process and associated complications. A number of therapeutic approaches and treatment combinations have been employed in the treatment of MM, relying primarily on high dose chemotherapy and autologous stem-cell transplantation, maintenance therapy using drug regimens such as alternate-day prednisone. However, with these approaches, the response rates and survival times did not differ between patients designated as either high or low-risk according to M protein values and the symptoms or presence of bone disease; and early treatment did not benefit asymptomatic subjects nor did delayed treatment improve treatment efficacy and survival. The increased ability to precisely identify prognostic factors such as cytogenic abnormalities and to determine risk has increased the individualization of treatment for MM, improving patient response and survival. The incorporation of immunomodulators and proteasome inhibitors such as bortezomib into treatment regimens has improved the survival of patients with MM. Treatment with thalidomide, however, is often associated with toxicity that limits its long-term use. Single-agent clinical activity of these newer drugs has been limited and most patients still relapse, so the search continues for more effective combinations of drugs or drugs with new mechanisms of action. In 2011, the multiple myeloma guidelines of the National Comprehensive Cancer Network introduced several combinations of drugs for primary induction therapy: 1) the combination of bortezomib/cyclophosphamide/dexamethasone for transplant candidates; 2) the combination of bortezomib/dexamethasone for patients who are not Luciferase does not undergo the significant post-translational modification within the ER that envelope glycoproteins candidates for transplantation; and the combination of melphalan/prednisone/lenalidomide for nontransplant candidates. Lenalidomide, an analogue of thalidomide, appears to be equally efficacious and less toxic than thalidomide. Lenalidomide differs from thalidomide by a single carbonyl ring and an amino acid group. Mechanistically, lenalidomide inhibits proliferation of tumor cells and induces apoptosis, as well as exerting immunomodulator effects, notably stimulating the production of cytokines and the activation of T cells and natural killer cells. Lenalidomide also has anti-angiogenic properties and is a particularly attractive option for maintenance treatment of MM. Indeed, a number of comprehensive review studies have reported positive findings regarding the use of lenalidomide in the treatment of MM in recent years. 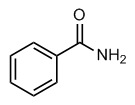 To gain a better, more complete understanding of the efficacy and safety of lenalidomide, we performed a meta-analysis of randomized controlled trials in which patients with MM received lenalidomide as initial or maintenance therapy. The methodological quality of the trials included in the metaanalysis is summarized in Table 2. Two trials reported acceptable methods of randomization.
To gain a better, more complete understanding of the efficacy and safety of lenalidomide, we performed a meta-analysis of randomized controlled trials in which patients with MM received lenalidomide as initial or maintenance therapy. The methodological quality of the trials included in the metaanalysis is summarized in Table 2. Two trials reported acceptable methods of randomization.