It is not clear whether additional component may exist to promote the cell killing process upstream of CED-3. Moreover, some cell death effectors that act downstream of CED-3, such as CED-8 and WAH-1, or are CED-3 substrates, such as DCR-1, are important for the timing or progression of programmed cell death. The eukaryotic translation initiation factor 3 plays Folinic acid calcium salt pentahydrate essential roles in the initiation of translation. The mammalian eIF3 complex contains 10�C13 subunits, including five highly conserved core subunits and five to eight less conserved non-core subunits. The 28 kDa human eIF3k protein was originally identified as a non-core subunit of the eIF3 complex. An in vitro reconstitution experiment showed that eIF3k is not required for the formation of the active eIF3 complex. Interestingly, eIF3k is conserved among metazoans, including C. elegans, D. melanogaster, M. musculus, and H. sapiens, but is absent in S. cerevisiae, suggesting a specialized role for eif-3.K in multicellular organisms. In addition, human eIF3k is associated with dynein, cyclin D3, the 5-HT7 receptor, and keratin K18, suggesting the involvement of eIF3k in processes that are unrelated to translation. Recently, we reported an apoptosis-promoting function for eIF3k in simple epithelial cells. Upon apoptotic stimuli, keratin K18 is cleaved by caspase 3, resulting in the collapse of K8/K18 intermediate filaments into apoptotic bodies and the sequestration of caspase 3 in kerain-containing inclusions. eIF3k binds to keratin inclusions, which in turn leads to the release of keratin-associated caspase into the cytosol to facilitate the execution of apoptosis. Keratin K8/K18 is the major intermediate filament in epithelial cells. 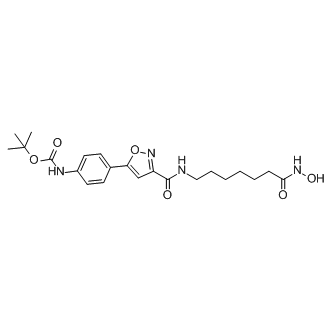 It is not clear whether eIF3k may potentiate Mechlorethamine hydrochloride apoptosis in other cell types, such as neurons or muscle cells, where intermediate filaments other than keratin are present. In addition, it is unclear whether the apoptosispromoting function of eIF3k has been conserved throughout evolution. In this work, we characterized the function of eif-3.K in C. elegans and showed that its apoptosis-promoting function has indeed been conserved throughout evolution. Furthermore, we identified a new function for the WH domain of EIF-3.K in the promotion of programmed cell death. We have previously shown that human eIF3k promotes apoptosis in cultured simple epithelial cells. In this work, we provide evidence that eif-3.K has a cell deathpromoting function at an organismal level and that this function has been conserved through evolution. In C. elegans, the loss of eif-3.K caused reduced programmed cell death and enhanced cell survival in sensitized mutants. In contrast, the overexpression of eif-3.K by the heat shock promoter or a touch neuron-specific promoter resulted in ectopic cell death. These results demonstrate that eif-3.K promotes programmed cell death. Our results also show that eif-3.K is essential for the efficient cell death that is induced by the overexpression of egl-1 or ced-4, but not ced-3, as the loss of eif-3.K partially suppresses the cell death that is induced by the overexpression of egl-1 or ced-4 only. In addition, the observation that ced-3 overexpression can rescue the cell death-defective phenotype of eif-3.K mutants and that the ced-3 strong mutation can suppress cell death caused by heat shock-induced eif-3.K overexpression further reinforces the notion that eif-3.K requires ced-3 to promote programmed cell death. Furthermore, the wide range in the identity and type of extraneous surviving cells that are affected by the eif-3.K mutation suggests that eif-3.K may be involved in the majority of programmed cell death.
It is not clear whether eIF3k may potentiate Mechlorethamine hydrochloride apoptosis in other cell types, such as neurons or muscle cells, where intermediate filaments other than keratin are present. In addition, it is unclear whether the apoptosispromoting function of eIF3k has been conserved throughout evolution. In this work, we characterized the function of eif-3.K in C. elegans and showed that its apoptosis-promoting function has indeed been conserved throughout evolution. Furthermore, we identified a new function for the WH domain of EIF-3.K in the promotion of programmed cell death. We have previously shown that human eIF3k promotes apoptosis in cultured simple epithelial cells. In this work, we provide evidence that eif-3.K has a cell deathpromoting function at an organismal level and that this function has been conserved through evolution. In C. elegans, the loss of eif-3.K caused reduced programmed cell death and enhanced cell survival in sensitized mutants. In contrast, the overexpression of eif-3.K by the heat shock promoter or a touch neuron-specific promoter resulted in ectopic cell death. These results demonstrate that eif-3.K promotes programmed cell death. Our results also show that eif-3.K is essential for the efficient cell death that is induced by the overexpression of egl-1 or ced-4, but not ced-3, as the loss of eif-3.K partially suppresses the cell death that is induced by the overexpression of egl-1 or ced-4 only. In addition, the observation that ced-3 overexpression can rescue the cell death-defective phenotype of eif-3.K mutants and that the ced-3 strong mutation can suppress cell death caused by heat shock-induced eif-3.K overexpression further reinforces the notion that eif-3.K requires ced-3 to promote programmed cell death. Furthermore, the wide range in the identity and type of extraneous surviving cells that are affected by the eif-3.K mutation suggests that eif-3.K may be involved in the majority of programmed cell death.