Recently, we have shown that 10058-F4 also reduces MYCN/MAX interaction in addition to c-MYC/MAX and that it induces selective apoptosis and cell growth arrest in MYCN-amplified compared to nonMYCN amplified NB cells. Furthermore we demonstrated significantly delayed tumor growth in a NB xenograft and increased survival in a transgenic mouse model of NB. These results are in contrast with a previous report that showed no significant antitumor activity of 10058-F4 in a xenograft model of prostate cancer. The differences might be due to the targeting of MYCN as well as c-MYC by 10058-F4 in NB, as well as to a potential greater reliance of NB cells on MYCN for their survival. Inspired by our earlier findings, we explored whether additional compounds shown to modulate c-MYC function could also bind MYCN and inhibit its function in NB cells. Here, extensive characterization of five compounds has been carried out including monitoring of their binding to MYCN as well as their effects in cellular assays used in our previous study. Our SPR analysis showed binding to both c-MYC and MYCN for all compounds tested except for the non-binder 7RH. The estimated KD values showed a similar affinity of 10058-F4 to both proteins. The approximate affinities we could determine were higher than the originally reported KD-values, which might be due to the fact that in the fluorescence polarization assay the bHLHZip domain is free in solution, while in the SPR-based assay it is immobilized on the chip surface by amine coupling and thus physically constrained. However we were encouraged by the fact that 10058-F4 also bound to MYCN with an approximately equal affinity as to cMYC. 10058-F4 is proposed to bind preferentially to Tyr402 and the hydrophobic region ARRY-142886 between residues 401 and 406 in c-MYC. As shown in Figure 1, MYCN also contains a Tyr at the analogous position as well as a highly homologous hydrophobic region. Taken together, these experiments suggest that the full biological effects of MYC inhibiting compounds are most apparent not only when the binding between MYCN and MAX is inhibited but when the levels of MYCN protein are reduced as well. Residual MYC activity may thus be sufficient to permit continued mitochondrial function, thus preventing the accumulation of neutral lipids. The serine hydrolase a/b-hydrolase domain containing 12 is a 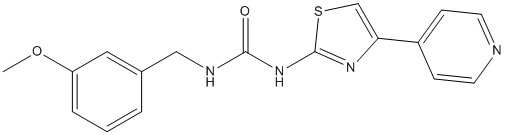 membrane-bound enzyme that together with monoacylglycerol lipase and ABHD6 contributes to the metabolism of the endocannabinoid 2-arachidonoylglycerol in vitro. In vivo, ABHD12 serves as a lysophospholipase showing preference towards lysophosphatidylserine in the mammalian nervous system. Even though ABHD12 is still poorly characterized, recently developed ABHD122/2 mice have shed some light to its possible physiological functions. In the study of Blankman et al., ABHD12 deficient mice developed age dependent symptoms that resemble the human neurodegenerative disorder PHARC. Authors suggested that the disrupted LPS metabolism and resulting neuroinflammation may form one of the molecular basis for PHARC. Tissue distribution and subcellular localization of MAGL, ABHD6 and ABHD12 are different, suggesting that these hydrolases could control different pools of 2-AG. An active site of ABHD12 is predicted to face the lumen and/or extracellular space and in the latter position ABHD12 could possibly metabolize extracellular pool of 2-AG. We have recently delineated the monoacylglycerol SJN 2511 substrate preferences of ABHD12 in vitro and found that unlike MAGL, ABHD12 prefers the 1-isomers of unsaturated MAGs over the 2isomers. More detailed pharmacological studies with ABHD12 have been limited due to the lack of selective inhibitor. Preliminary inhibitor profiling has shown that the universal lipase/serine hydrolase inhibitors tetrahydrolipstatin and methyl arachidonyl fluorophosphonate.
membrane-bound enzyme that together with monoacylglycerol lipase and ABHD6 contributes to the metabolism of the endocannabinoid 2-arachidonoylglycerol in vitro. In vivo, ABHD12 serves as a lysophospholipase showing preference towards lysophosphatidylserine in the mammalian nervous system. Even though ABHD12 is still poorly characterized, recently developed ABHD122/2 mice have shed some light to its possible physiological functions. In the study of Blankman et al., ABHD12 deficient mice developed age dependent symptoms that resemble the human neurodegenerative disorder PHARC. Authors suggested that the disrupted LPS metabolism and resulting neuroinflammation may form one of the molecular basis for PHARC. Tissue distribution and subcellular localization of MAGL, ABHD6 and ABHD12 are different, suggesting that these hydrolases could control different pools of 2-AG. An active site of ABHD12 is predicted to face the lumen and/or extracellular space and in the latter position ABHD12 could possibly metabolize extracellular pool of 2-AG. We have recently delineated the monoacylglycerol SJN 2511 substrate preferences of ABHD12 in vitro and found that unlike MAGL, ABHD12 prefers the 1-isomers of unsaturated MAGs over the 2isomers. More detailed pharmacological studies with ABHD12 have been limited due to the lack of selective inhibitor. Preliminary inhibitor profiling has shown that the universal lipase/serine hydrolase inhibitors tetrahydrolipstatin and methyl arachidonyl fluorophosphonate.