Emerging data indicated that oxidative stress due to increased AbMole Lomitapide Mesylate reactive oxygen species generation and/or compromised antioxidant systems represents an important factor in the progression of insulin resistance. One of the main sources of ROS is NADPH oxidase, a multi-protein enzyme complex that uses NADPH as a substrate to convert molecular oxygen to ROS. Components of NADPH oxidase complex of phagocytes include the membrane-bound cytochrome b558, composed of 2 subunits, p22-phox and gp91-phox, and 4 cytosolic subunits, p47-phox, p67-phox, p40-phox, and the small GTP-binding protein, Rac. Moreover, expression of gp91phox, p22phox, p40 phox, p47phox, and p67phox have been documented in skeletal muscle. Wei et al found NADPH oxidase activation and ROS generation play an important role in Ang II-induced inhibition of insulin signaling in skeletal muscle cells. Given these data, studies are warranted to ascertain whether UII mediates skeletal muscle IR by increasing ROS production via NADPH oxidase. In the present study, we sought to determine whether UII antagonism improved glucose tolerance by decreasing the oxidative state in KK mice, and to investigate the effect of UII on ROS production and on glucose transport signaling in C2C12 mouse myotube cells. Insulin resistance in peripheral tissues is an essential element in the pathogenesis of T2DM. The KK mouse, an 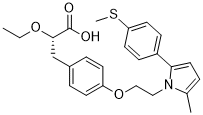 animal model of T2DM, is characterized by insulin resistance, hyperglycemia and hyperinsulinemia. In the present study, we demonstrated for the first time that urantide administration improves glucose tolerance in this mouse model. Furthermore, urantide treatment decreased the level of MDA, as well as p67phox, p40-phox translocation, and increased the phospho-form of AKT, PKC and ERK in the skeletal muscle of the KK mouse. These results indicated that urantide may improve skeletal muscle glucose transport signaling pathways by decreasing ROS production via NADPH oxidase. In order to further study the effect of UII on ROS production and the signaling pathways involved in glucose transport, we evaluated the effect of UII on ROS production, NADPH oxidases subunits AbMole Gemifloxacin mesylate translocations, and AKT/PKC/ERK/P38MAPK phosphorylation in C2C12 cells. Pretreatment with 100 nM UII increased ROS production, NADPH oxidases subunits translocations, and decreased AKT/PKC/ERK phosphorylation. Taken together, these data suggest that UII can impair skeletal muscle glucose transport signaling pathways by increasing ROS production, and that urantide can ameliorate these effects. ROS plays an important role as signaling molecules in skeletal muscle IR, and NADPH oxidases are known to be involved in the ROS production in skeletal muscle.
animal model of T2DM, is characterized by insulin resistance, hyperglycemia and hyperinsulinemia. In the present study, we demonstrated for the first time that urantide administration improves glucose tolerance in this mouse model. Furthermore, urantide treatment decreased the level of MDA, as well as p67phox, p40-phox translocation, and increased the phospho-form of AKT, PKC and ERK in the skeletal muscle of the KK mouse. These results indicated that urantide may improve skeletal muscle glucose transport signaling pathways by decreasing ROS production via NADPH oxidase. In order to further study the effect of UII on ROS production and the signaling pathways involved in glucose transport, we evaluated the effect of UII on ROS production, NADPH oxidases subunits AbMole Gemifloxacin mesylate translocations, and AKT/PKC/ERK/P38MAPK phosphorylation in C2C12 cells. Pretreatment with 100 nM UII increased ROS production, NADPH oxidases subunits translocations, and decreased AKT/PKC/ERK phosphorylation. Taken together, these data suggest that UII can impair skeletal muscle glucose transport signaling pathways by increasing ROS production, and that urantide can ameliorate these effects. ROS plays an important role as signaling molecules in skeletal muscle IR, and NADPH oxidases are known to be involved in the ROS production in skeletal muscle.