Protein C inhibitor is a serine protease inhibitor and a member of the serpin superfamily. PCI has originally been described as a plasma inhibitor of activated protein C. Later, the inhibition of several other proteases, including the pancreatic enzymes trypsin and chymotrypsin, by PCI has been shown.. Like other members of the serpin family, PCI acts as a suicide substrate for its target proteases. Serpins have an exposed reactive center loop which offers a potential cleavage site for the protease. The protease recognizes this sequence and binds to the serpin, forming a reversible Michaelis-like complex. Then the protease cleaves the reactive site peptide bond and the serpin incorporates the RCL into b-sheet A, producing a covalent serpin-protease complex. The enzymeinhibitor complex can dissociate, leaving behind an active protease and a cleaved, inactive serpin. Heparin and other glycosaminoglycans can modify the activity and target enzyme specificity of PCI. The heparin-binding site is a basic patch on helix H, which lies close to the reactive center loop. Heparin changes the 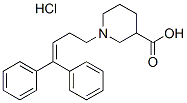 charge of this area, thereby modifying the affinity of PCI towards different proteases. Heparin stimulates the inhibition of APC and thrombin, but abolishes the inhibition of tissue kallikrein by PCI. Antithrombin, GDC-0879 another heparin-binding serpin, uses a different mechanism. Both low molecular weight and unfractionated heparin bind to helix D. This binding leads to a conformational change of AT and an additional part of the reactive center loop is exposed. This results in increased inhibition of coagulation proteases. UFH is furthermore big enough to span from helix D to the protease. It thereby forms a template for AT and thrombin and enhances their interaction. By Northern blotting, a wide tissue distribution of PCI has been demonstrated in humans. PCI mRNA is present in the liver, kidney, heart, brain, lung, spleen, reproductive system and pancreas. Radtke et al. showed by in situ hybridization that PCI is expressed in the exocrine part of the pancreas, and by Western blotting that the protein is present in pancreatic fluid. We have shown that PCI mRNA and protein are also present in keratinocytes of the human skin. Its expression is increased in the more differentiated layers of the epidermis. PCI is also present in several body fluids and secretions, e.g. in plasma and seminal fluid. In rodents, PCI is almost exclusively present in the reproductive tract. This makes it difficult to study the effect of PCI outside the reproductive tract in animal models. Because of its wide tissue distribution, PCI may have several functions in humans. So far, very little is known about these functions. PCI might have a protective effect against cancer progression. Since PCI has affinity for glycosaminoglycans and phospholipids, both Y-27632 dihydrochloride components of the cell membrane, cell membrane association of PCI is not unlikely. We were therefore interested in analyzing the interaction of PCI with serine proteases also present in or on cell membranes. So far there are only a few indications in the literature, suggesting that PCI interacts with type II transmembrane serine proteases. However, as far as inhibition kinetics or the effect of glycosaminoglycans or phospholipids is concerned, no data is available on these interactions. It was therefore the aim of this study to analyze the interaction of PCI with enteropeptidase. EP is a type II transmembrane serine protease, located mainly at the brush border membrane of the epithelial cells of the duodenum and jejunum. Active EP also occurs in duodenal fluid. In the small intestine, EP activates trypsinogen to trypsin. Active human EP is composed of a light and a heavy chain linked by a disulfide bond.
charge of this area, thereby modifying the affinity of PCI towards different proteases. Heparin stimulates the inhibition of APC and thrombin, but abolishes the inhibition of tissue kallikrein by PCI. Antithrombin, GDC-0879 another heparin-binding serpin, uses a different mechanism. Both low molecular weight and unfractionated heparin bind to helix D. This binding leads to a conformational change of AT and an additional part of the reactive center loop is exposed. This results in increased inhibition of coagulation proteases. UFH is furthermore big enough to span from helix D to the protease. It thereby forms a template for AT and thrombin and enhances their interaction. By Northern blotting, a wide tissue distribution of PCI has been demonstrated in humans. PCI mRNA is present in the liver, kidney, heart, brain, lung, spleen, reproductive system and pancreas. Radtke et al. showed by in situ hybridization that PCI is expressed in the exocrine part of the pancreas, and by Western blotting that the protein is present in pancreatic fluid. We have shown that PCI mRNA and protein are also present in keratinocytes of the human skin. Its expression is increased in the more differentiated layers of the epidermis. PCI is also present in several body fluids and secretions, e.g. in plasma and seminal fluid. In rodents, PCI is almost exclusively present in the reproductive tract. This makes it difficult to study the effect of PCI outside the reproductive tract in animal models. Because of its wide tissue distribution, PCI may have several functions in humans. So far, very little is known about these functions. PCI might have a protective effect against cancer progression. Since PCI has affinity for glycosaminoglycans and phospholipids, both Y-27632 dihydrochloride components of the cell membrane, cell membrane association of PCI is not unlikely. We were therefore interested in analyzing the interaction of PCI with serine proteases also present in or on cell membranes. So far there are only a few indications in the literature, suggesting that PCI interacts with type II transmembrane serine proteases. However, as far as inhibition kinetics or the effect of glycosaminoglycans or phospholipids is concerned, no data is available on these interactions. It was therefore the aim of this study to analyze the interaction of PCI with enteropeptidase. EP is a type II transmembrane serine protease, located mainly at the brush border membrane of the epithelial cells of the duodenum and jejunum. Active EP also occurs in duodenal fluid. In the small intestine, EP activates trypsinogen to trypsin. Active human EP is composed of a light and a heavy chain linked by a disulfide bond.